Dessolve III - Study news
Newsletter July 2015
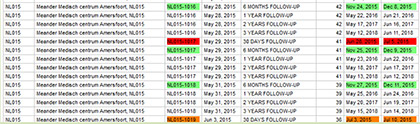
NEW eCRF TOOL FOLLOW UP PLANNING
Planning the follow-up visits for all of your patients, within the window can be a challenge. There is now a very handy tool for this in the eCRF. Please go to the ‘Reports’ tab and select the ‘FUP PLANNER’.
In the overview you can find per patient (patient ID) and per follow-up visit (visit) the suggested FROM – TO date range in which a visit should be performed. Colors indicate if the visit should be planned with the patient (green), is within its window (orange) or if the visit is overdue and should be performed as soon as possible (red).
Once you perform the visit and enter the data in the eCRF the visit no longer shows in this FUP planner. The overview can also be exported to Word, Excel or PDF by clicking on the export button at the bottom of the page.

ALL COMERS DESIGN by M. Vorage (Cardialysis)
The “all-comers” design reflects the routine clinical practice of PCI with limited exclusion criteria. The “all-comers” design of the Dessolve-III study implies the enrolment of an unrestricted patient population to be treated with a MiStent or a Xience stent. This can be achieved by taking the following aspects into account at your site: 1) kick-off meeting to inform the whole department about the Dessolve-III trial and addressing the “all-comers” design ; 2) have worksheets ready prior to the initiation visit; 3) have sufficient study materials in-house prior to enrolment like stents, and also maintaining sufficient supply (e.g. re-supply of MiStents via the eCRF); 4) enable excellent teamwork between interventional cardiologists, fellows (clinical + research), wards of admission & study coordinators including appointed back-up for study specific procedures (e.g. screening, interventions, eCRF completion, SAE reporting, patient follow-up etc.).
Newsletter June 2015
SCIENTIFIC BULLETIN by T. Engels (Micell)
“DESSOLVE I and II were both designed to follow patients through 5yrs of followup. DESSOLVE I (30 patients, 5 sites, FIM study) 4yrs results were recently presented at the EuroPCR, and the data continues to be very encouraging. Adjudicated MACE (Death, MI, TVR) rate at 4 years is 10.3%; the target lesion revascularization (TLR) rate is 0.0%; and the stent thrombosis rate is 0.0%. The CE Mark approval trial, DESSOLVE II (184 patients, 26 sites, 2:1 randomization against Endeavor), is currently completing 4yrs follow-up visits with results to be presented later this year”.
FREQUENTLY ASKED QUESTIONS
Q: Can the study MiStent be used for a re-PCI?
A: No, the MiStent provided for the study should only be used for the index procedure & staged procedure. For a re-PCI, use any stent that your hospital purchased.
Q: What kind of stent is preferred to use for a re-intervention when a MiStent was implanted during the index procedure.
A: Dependent on the stents left on the shelf, a Xience/ drug eluting stent is preferred
Q: For referral patients it may be difficult to return for the 1 Month visit. How to deal with this?
A: Patients can go to their referral hospital for the 1 Month visit, and the ECG can be done there and sent to the enrolling site
Newsletter May 2015
SCIENTIFIC BULLETIN by prof de Winter on MiStent (Micell)
“During initial experience with the MiStent in the DESSOLVE III study, the device performed well, with good profile, excellent deliverability and trackability. Positioning was easy, visibility was good. The stent was expanded and angiographic result was excellent”.
IMPORTANT INFORMATION
Cardiac biomarkers 6 hours post-PCI
As Myocardial Infarctions (MIs) represent about 40% part of the primary endpoint (TLF), it is therefore of the utmost importance to collect all cardiac biomarkers as per protocol. The peri-procedural MIs (and also the proof of no peri-procedural MIs) largely depend on the post procedure cardiac biomarkers. Furthermore, the Clinical Event Committee adjudicates according to 3 MI definitions, in order to compare event reporting between other (historical) trials. In order to do this CK, CKMB and Troponin are all required.
Patient file
Sites are requested to stick the ‘peel-off’ label (present on stent box and pouch) in the patient file due to unique device identifier tracking.
FREQUENTLY ASKED QUESTIONS
Q: Can patients on oral anticoagulation (e.g. coumarin) be included?
A: Yes. We recommend you to follow dose/regimen according to 2014 ESC/EACTS Guidelines on myocardial revascularization.
Q: Can the study MiStent be used for a re-PCI?
A: No, the MiStent provided for the study should only be used for the index procedure & staged procedure. For a re-PCI, use any stent that your hospital purchased.
Q: Is it allowed to include patients with a bifurcation?
A: Yes
Q: For referral patients it may be difficult to return for the 1 Month visit. How to deal with this?
A: Patients can go to their referral hospital for the 1 Month visit, and the ECG can be made there.
Q: MiStents should overlap at least 5 mm according to the protocol. Should that not be as minimal as possible?
A: Yes 1-2 mm overlap is sufficient, depending on the lesion and the location, judged by the operator