GALILEO 4D - STUDY DESIGN
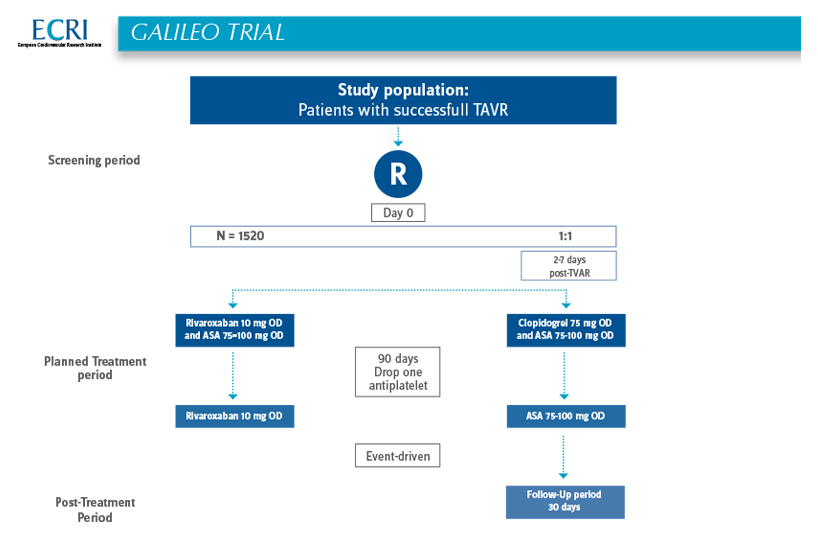
The GALILEO-4D trial will be conducted as a substudy of the multicenter, open-label, randomized, event-driven, active-controlled GALILEO trial. Patients will be 1:1 randomized to an antiplatelet-based strategy vs. rivaroxaban-based strategy – the randomization will adopt the same 1:1 randomization of the main GALILEO trial. In case the GALILEO-4D trial should still be continued after completion of the main GALILEO trial, this 1:1 randomization will be continued until inclusion of 150 patients in both treatment groups. In total, 300 patients will be randomized in the GALILEO-4D trial.
The 4DCT-scan should preferentially be performed at the same day of Visit number 3 of the main GALILEO trial (90 days ± 15 days after randomization). Time window for the 4DCT-scan is from 15 days before Visit number 3 up to the day of Visit number 3.