Global leaders
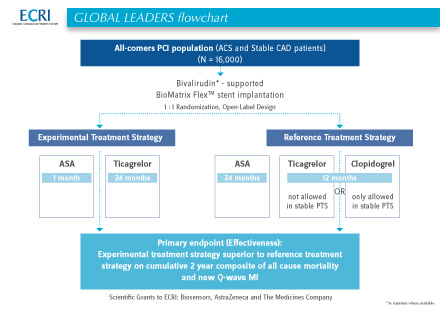
The GLOBAL LEADERS trial was an Investigator-Initiated Study (IIS) in which 16,000 “all-comer” patients were enrolled in 130 centers in 18 countries in Europe, Australia, Brazil, Canada & Singapore. Patients treated with bivalirudin (in countries where available) and a Biomatrix stent have been randomized either to ticagrelor plus aspirin, followed by 1 month of ticagrelor monotherapy for 23 months, or intensive dual antiplatelet therapy for 24 months.
The first patient in the GLOBAL LEADERS trial was enrolled by Dr. Aminian from the CHU in Charleroi, Belgium.
GLOBAL LEADERS run under the umbrella of the European Cardiovascular Research Institute, ECRI (http://clinicaltrials.gov/show/NCT01813435). ECRI was supported by scientific grants from Biosensors, AstraZeneca, and The Medicines Company.
In this important trial, Cardialysis was responsible for project management, data management, site monitoring, the Data Safety and Monitoring Board, safety reporting, and ECG analysis. The GLOBAL LEADERS trial was published in The Lancet in 2018.